In this post, you’ll learn about the performance of boiler, efficiency, power and heat loss with simple equations and heat balance sheet.
Performance of Boiler
The performance of boiler is measured in terms of its evaporative capacity. However, the evaporative capacities of two boilers cannot be compared unless both the boilers have the same feed water temperature, working pressure, fuel and the final condition of steam.
In actual practice, the feedwater temperature and working pressure vary considerably. It is thus obvious, that the comparison of two boilers becomes difficult unless some standard feed temperature and working pressure are adopted.
The feed temperature usually adopted is 100°C and the working pressure as normal atmospheric pressure, i.e., 1.033 kg/cm². It is assumed that the boiler is supplied with water at the boiling temperature (100° C) corresponding to the atmospheric pressure.
Read also:
- Steam Boilers: Parts, Types, Classification, Advantages, Application, and More
- Boiler Mountings and Accessories: Types, and Their Working.
Equivalent Evaporation
It is the amount of water evaporated from feed water at 100° C and formed into dry and saturated steam at 100º C at normal atmospheric pressure. It is written as “from and at 100°C”.
As the water is already at the boiling temperature, it requires only latent heat at 1.033 kg/cm2 to convert it into steam at the temperature (100° C). The value of this latent heat is taken as 539.0 kcal/kg. Mathematically,
Equivalent evaporation “from and at 100° C”,
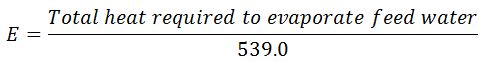
Let
- t1 = Temperature of feed water in ° C,
- h1 = Sensible heat of feed water in kcal/kg of steam corresponding to t1°C,
- H = Total heat of steam in kcal/kg of steam at a given working pressure
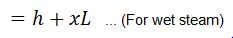
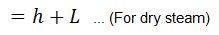
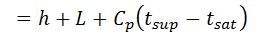
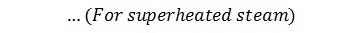
We = Weight of water actually evaporated or steam produced in kg/hr or kg/kg of fuel burnt.
We know that heat required to evaporate 1 kg of water
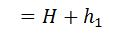
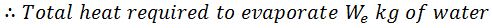
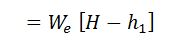
And
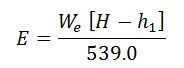
Note:
The factor is known as factor of evaporation and is usually denoted by Fe. Its value is greater than unity for all boilers.
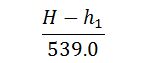
In S.I. units, the value of latent heat at a temperature of 100° C is 2256.9 KJ/kg.
Boiler Efficiency
It may be defined as the ratio of heat actually used in producing the steam to the heat liberated in the furnace. It is also known as the thermal efficiency of the boiler.
Mathematically,
Boiler efficiency or thermal efficiency,
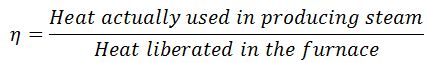
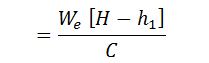
Where
- We = Weight of water actually evaporated or actual evaporation in kg/kg of fuel, and
- C = Calorific value of fuel in kcal/kg of fuel.
Note:
If We = Total weight of water evaporated into steam in kg, and Wf = Weight of fuel used in kg.
Then
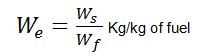
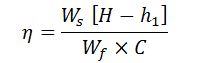
If the boiler contains an economiser & superheater is considered a single unit, then the efficiency is termed as the overall efficiency of the boiler.
Boiler Power
The American Society of Mechanical Engineers (ASME) has recommended that one boiler horsepower is equivalent to evaporation of 15653 kg of water per hour from and at 100° C.
Mathematically :
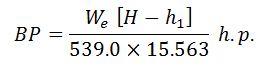
where
- We = Weight of water actually evaporated,
- H = Total heat of the steam formed, and
- h1 = Sensible heat of feed water.
Boiler Trial
The purposes of a boiler trial are:
- Used to Determine the generating capacity of the boiler.
- To determine the thermal efficiency of the boiler when working at a certain pressure.
- To prepare the heat balance sheet for the boiler.
We have already discussed the first two objects in the previous articles. Now we shall discuss the third object, i.e. to prepare the heat balance sheet.
Heat Losses in a Boiler
We know that the efficiency of a boiler is the ratio of heat used in producing steam to the heat released in the furnace. Also, heat utilised is always less than the heat liberated in the furnace.
The difference of heat liberated in the furnace and heat utilised in producing steam is known as heat loss in the boiler. The loss of heat may be divided into various heads, but the following are important from the subject point of view :
1. Heat loss in dry flue gases
Heat lost to dry flue gases/kg of fuel
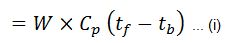
Where
- We = weight of dry flue gases per kg of fuel,
- Cp = Mean specific heat of dry exhaust gases,
- tf = Temperature of flue gases leaving chimney,
- tb = Temperature of the boiler room.
This loss is maximum in a boiler.
2. Heat loss in moisture present in the fuel
It is assumed that the moisture is converted into superheated steam at atmospheric pressure (1.033 kg/cm).
Heat loss in moisture present in the fuel
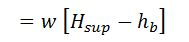
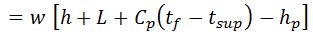
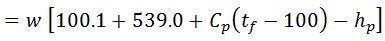
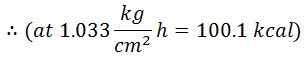
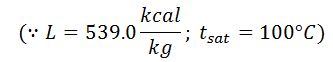
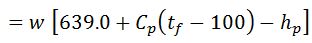
Where
- w = Weight of moisture per kg of fuel,
- Cp = Mean specific heat of superheated steam in exhaust gases,
- tf = Temperature of flue gases leaving chimney,
- tb = Temperature of the boiler room, and
- hb = Sensible heat of water at boiler room temperature.
3. Heat lost to steam formed by combustion of hydrogen/kg of fuel
Let
H2 = weight of hydrogen present per kg of fuel.
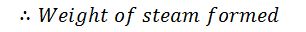
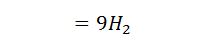
Then the heat lost to steam/kg of fuel
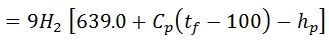
Where w is the weight of moisture per kg of fuel
Note:
Heat lost to steam & moisture/kg of fuel
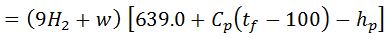
4. Heat loss due to unburnt carbon is ash pit
The heat loss due to unburnt carbon per kg of fuel
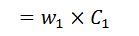
Where
- w1 = Weight of carbon in ash pit per kg of fuel.
- C1 = Calorific value of carbon.
5. Heat loss due to incomplete combustion of carbon to carbon monoxide (CO)
This loss occurs in the boiler due to insufficient air supply.
Heat loss due to incomplete combustion
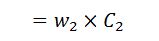
Where
- w2 = weight of carbon monoxide in flue gas per kg of fuel, and
- C2 = Calorific value of carbon monoxide.
6. Heat loss due to radiation
There is no direct method for finding the beat lost due to radiation. This loss is calculated by subtracting the heat used in increasing steam and heat losses from the heat supplied.
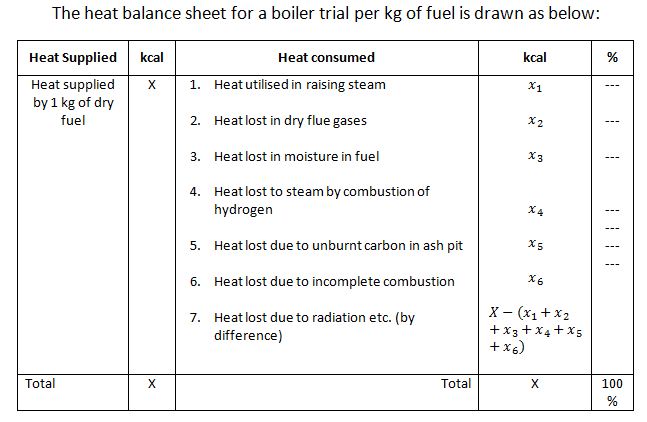
Heat Balance Sheet
A heat balance sheet shows the complete account of heat supplied by 1 kg of dry fuel and heat consumed. The heat supplied is used for increasing the steam and the remaining heat is lost. We know that heat used in increasing steam/kg of fuel,
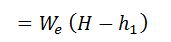
Read Next:
- Cochran Boiler: Parts, Working, Advantages, Mountings and More
- Babcock And Wilcox Boiler: Parts, Mountings, Working, Advantages and More
- Locomotive Boiler: Parts, Construction, Working, Advantages and Applications
That’s it, thanks for reading. If you have any questions about “performance of boiler” ask in the comments below. If you found this post helpful share with your friends.
i am excited to follower of this site the pdf form it is easy to read and dowload keep it up.
Thanks for your feedback. Keep visiting.