Hello readers! In this post, we’ll discuss What is Thermodynamics and the Four Laws of Thermodynamics using illustrations.
What is Thermodynamics?
This article is about the laws of thermodynamics. Thermodynamics is a branch of the science of energy that transmits heat from one form to another.
It mainly deals with heat and work energy and their effect on the properties of substances. Thermodynamics comes from the Greek words, thermal means heat, and dynamics means power.
The study of thermodynamics started with analyzing heat engine processes to improve engine efficiency. Today its scope is widened and there are important applications of the thermodynamics principles outside the field of heat engines.
Branches of Thermodynamics
There are four branches of thermodynamics:
Classical Thermodynamics
The behavior of matter is analyzed from a macroscopic view is known as classical thermodynamics. To determine additional qualities and estimate the features of the matter conducting the process, units like temperature and pressure are taken into account.
Statistical Thermodynamics
Every molecule is examined in statistical thermodynamics, meaning that the characteristics of each molecule and their interactions are taken into account to describe the behavior of a collection of molecules.
Chemical Thermodynamics
The study of the relationship between heat and work in chemical processes and changing states is known as chemical thermodynamics.
Equilibrium Thermodynamics
The study of energy and matter changes as they get closer to equilibrium is known as equilibrium thermodynamics.
Read Also: List of Mechanical Properties That Every Mechanical Engg
Concept of Thermodynamics
There are many terms associated with thermodynamics, each with its meaning. Understanding the basic concepts prevents misunderstandings about various topics discussed in thermodynamics.
System
A thermodynamic system is a defined portion of matter with a clear boundary on which we focus our attention. The boundary of the system could be fake or actual, fixed or flexible.
Types of Systems:
- Isolated System: An isolated system is incapable of exchanging mass and energy with its surroundings. The universe is an example of an isolated system.
- Closed System: The transfer of energy occurs over the boundary of the closed system, but mass is not transferred. Closed systems examples include refrigerators and the compression of gas in the piston-cylinder assembly.
- Open System: In an open system, mass and energy can both be transmitted between the system and its surroundings. One example of an open system would be a steam turbine.
Surrounding
A surrounding is anything that exists outside of the system that directly affects how the system behaves.
Thermodynamic Process
A thermodynamic process occurs when there is an energy change within a system that results in changes in pressure, volume, and internal energy. Thermodynamic processes are classified into four types with different properties:
- Adiabatic Process – It is a process that does not allow heat to enter or exit the system.
- Isochoric Process – It is a process in which the volume does not change and the system does not perform any work.
- Isobaric Process – It is a process that does not result in any pressure changes.
- Isothermal Process – It is a process that does not result in any temperature changes.
Thermodynamic Cycle
A thermodynamic cycle is a set of operations carried out in such a way that the system’s initial and final states are the same. Thermodynamic cycles are often referred to as cyclic operations or cyclic processes.
Thermodynamic Equillirium
At a particular state, all a system’s properties are fixed. Therefore, when the value of even one property changes, the system’s state changes.
When a system is in equilibrium, its qualities remain unchanged even when it is separated from its surroundings.
- Thermal equilibrium is achieved when the temperature remains constant throughout the system.
- Mechanical equilibrium occurs when there is no change in pressure at any point in the system.
- Chemical equilibrium is defined as a system whose chemical composition does not change over time.
Thermodynamic Properties
The thermodynamic properties are defined as a system’s unique characteristics that can be used to specify its state. Thermodynamic properties can be either extensive or intensive.
- Intensive properties are those that do not depend on the amount of substance. Two intense properties are temperature and pressure.
- When it comes to extensive properties, the mass of the system determines their values. Three extensive properties are volume, energy, and enthalpy.
Enthalpy and Entropy
What is Enthalpy?
In a thermodynamic system, enthalpy is the measure of energy. Enthalpy is the overall heat content of a system, which is equal to the system’s internal energy plus the volume times the pressure.
It is given by,
H = E + PV
The enthalpy, H, is calculated by adding the internal energy, E, and the product of the system’s pressure, P, and volume, V.
What is Entropy?
Entropy is a quantity that varies with the physical state of a system. In other terms, it’s a thermodynamic function that measures randomness or chaos.
In a solid, where the particles are not free to move, the entropy is lower than in a gas, where the particles fill the container.
Laws of Thermodynamics
There are three laws of thermodynamics:
- Zeroth law of thermodynamics
- First law of thermodynamics
- Second law of thermodynamics
- Third law of thermodynamics
Zeroth law of Thermodynamics
It states that
”when two bodies are in equilibrium with a third body, then they are also in thermal equilibrium with each other.
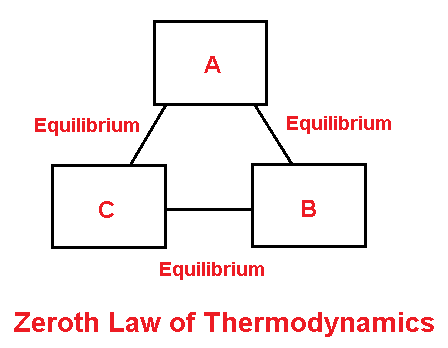
For example, if body AB is in thermal equilibrium with body C, then A & B must be in thermal equilibrium with each other.
First Law of Thermodynamics
- It states that ”the heat and work are mutually convertible”. it is the law of conservation of energy.
- i.e., energy can neither be created nor destroyed, but it can be converted into another form of energy.
- If a thermodynamic system is operating in a closed cycle, then the heat transfer is directly proportional to the work transfer.
- Mathematically according to this law, we have
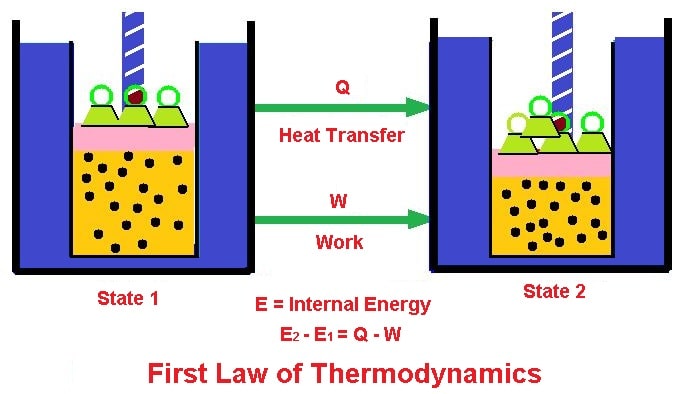
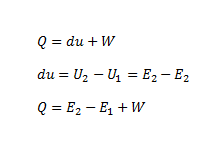
where,
- Q = Heat supplied
- W = external work done
- du = change in internal energy
Second Law of Thermodynamics
This law states.
” There is a definite limit to the amount of mechanical energy, which can be obtained from a given quantity of heat energy.”
It is explained by using two different statements,

Clausius statement – This law of thermodynamics has been enunciated by Clausius in a slightly different form, as
“it is impossible for a self-acting machine working in a cyclic process, to transfer heat from a body at low temperature to a body at a high temperature without the external use or heat cannot flow from a cold body without use”.
In other words, heat cannot flow from a cold body to a hot body without the help of an external agency.
Kelvin-Planck statement – It states that
“It is impossible to construct an engine working on a cyclic process, whose purpose is to convert all heat energy into work”.
In other words, no actual heat engine, working on a cycle process, can convert the heat energy supplied to it into mechanical work. It means that there is a degradation of energy in the process of producing mechanical work from heat. According to this statement, the second law of thermodynamics is sometimes called the law of degradation of energy.
Third Law of Thermodynamics
According to the third laws of thermodynamics, when a system’s temperature approaches absolute zero, its entropy approaches a constant value.
At absolute zero temperature, a pure crystalline material (perfect order) has zero entropy. There is only one state of a perfect crystal with minimum energy, according to this statement.
Third Law Of Thermodynamics Examples:
- Its molecules are highly entropy and move freely.
- When the temperature drops below 100 °C, the steam turns into water, where molecules can’t move as freely, lowering the entropy of the liquid.
- When water gets chilled below 0 degrees Celsius, it solidifies as ice. In this condition, molecular movement is increasingly restricted and the system’s entropy decreases.
- The molecules’ ability to move about inside the ice is increasingly restricted as their temperature drops, which results in a continuous decrease in the substance’s entropy.
Examples of Thermodynamics in Everyday Life
Thermodynamics is used everywhere, whether we are traveling in a car or relaxing in an air-conditioned hotel. Many different kinds of transportation, including trucks, ships, and airplanes, operate based on the second law of thermodynamics.
Thermodynamics serves as the foundation for all three of the heat transmission modalities. Heat transfer ideas are commonly employed in radiators, heaters, and coolers.
Thermodynamics is used in the study of several types of power plants, including nuclear and thermal power plants.
Summary
So now, I hope I’ve covered everything about the “Laws of thermodynamics“. If you have any questions or doubts about this article, you can ask in the comments. If you liked this article, then please share it with your friends.
Want free PDFs direct to your inbox? Then subscribe to our newsletter.
Read more on this blog:
- Shaper machine and types of shaper machine
- Types of micrometer screw gauge
- Reciprocating pump and working principle.
FAQs
The study of heat, work, and temperature in connection to energy, entropy, and the physical characteristics of matter and radiation is known as thermodynamics.
The first law of thermodynamics states that energy cannot be generated or destroyed. The second law of thermodynamics states that when a spontaneous event occurs, the universe’s entropy increases. The third law of thermodynamics states that an ideal crystal at zero Kelvin has zero entropy.
The laws of thermodynamics specify physical quantities such as temperature, energy, and entropy, which characterize thermodynamic systems in thermal equilibrium.