In this article, you will learn about different types of batteries with their working & applications are explained with Pictures.
If you need a PDF file? Just download it at the end of the article.
What is a Battery?
A battery is a device that holds electrical energy in the form of chemicals. An electrochemical reaction converts stored chemical energy into electrical energy (DC).
The electrochemical reaction in a battery is carried out by moving electrons from one material to another (called electrodes) using an electric current. The first battery was invented in 1800 by Italian physicist Alessandro Volta.
Whether you are an engineer or not, you must have seen at least two different types of batteries that is small batteries and larger batteries. Smaller batteries are used in devices such as watches, alarms, or smoke detectors, while applications such as cars, trucks, or motorcycles, use relatively large rechargeable batteries.
Batteries have become a significant source of energy over the past decade. Moreover, batteries are available in different types and sizes as per their applications. We will discuss different types of batteries and their uses, so let’s get started.
Read Also: Different Types of Fasteners and Their Uses & Examples
How Does A Battery Work?
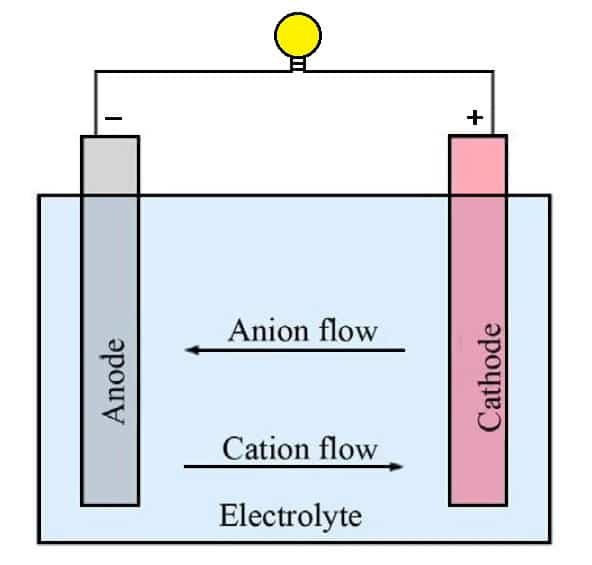
The battery produces electrical energy on demand by using the terminals or electrodes of the battery. The positive terminal is located on the top of the battery which is used for customer interests such as flashlights and electronics.
The outer case or bottom of the battery is commonly referred to as the negative terminals. Both terminals are very common in all types of batteries. The chemicals that surround these terminals and the battery together form the power cell.
The power cell generates energy whenever the positive and negative terminals are connected to an electrical circuit. For example, the metal part in the flashlight case and the device is on. The chemicals inside the cell (alkaline or lithium) begin a reaction to produce the ions and electrons that power anything attached to the battery.
Read Also: What is an Insulator? How does it work In transmission lines?
Classification of Batteries
- Primary battery
- Secondary battery
#1 Primary Battery
A primary battery is a simple and convenient source of electricity for many portable electronic devices such as lights, cameras, watches, toys, radios, etc. These types of batteries cannot be recharged once they are exhausted. They are composed of electrochemical cells whose electrochemical reactions cannot be reversed.
Generally, primary batteries are relatively inexpensive, lightweight, and convenient to use, with little or no maintenance. Primary batteries exist in many sizes and forms, ranging from coin cells to AA batteries. These are commonly seen in applications like pacemakers, animal trackers, wristwatches, remote controls, children’s toys, etc.
#2 Secondary Battery
Secondary batteries use electrochemical cells whose chemical reactions can be reversed by applying a certain voltage to the battery. It is also known as a rechargeable battery because it can be recharged after the battery’s energy is depleted. They are used as inverters for power supply as well as standalone power sources.
They are also used where it would be too expensive or impractical to use a single charged battery. Small-capacity secondary batteries are used in portable devices such as mobile phones, while heavy-duty batteries are found in electric vehicles and other high-drain applications.
Types of Batteries

The following are the types of batteries that are explained with their uses:
- Lead-acid batteries
- Nickel-cadmium batteries (Ni-Cd)
- Nickel-metal hybrid batteries (Ni-MH)
- Lithium-ion batteries (Li-ion)
- Alkaline batteries
- Zinc-carbon batteries
- Coin cell batteries
- Zinc-air cells
- Sealed lead-acid batteries
Read Also: What are the different types of DC generators?
#1 Lead-acid Batteries

It is a type of rechargeable battery containing lead acid that is much cheaper and is seen in most cars and vehicles to power the lighting system. Lead-acid batteries have a relatively low energy density compared to modern rechargeable batteries.
Despite this, their ability to supply high currents means that the cells have a relatively large power-to-weight ratio. Lead-acid battery capacity is 2V to 24V and is commonly seen as 2V, 6V, 12V, and 24V batteries. Its power density is 7 Wh/kg.
Since they are available at a low cost, providing the high current required by starter motors makes them perfect for use in motor vehicles.
#2 Nickel-cadmium Batteries (Ni-Cd)

Nickel-cadmium battery is also a type of rechargeable battery that uses nickel oxide hydroxide and the metal cadmium as electrodes. One of the main advantages of Ni-Cd batteries is that they can maintain voltage and hold a charge when not in use.
These types of batteries have a terminal voltage that drops almost to the end of the discharge during a discharge of about 1.2 volts. Although they are rarely used, they are cheap and have a much lower discharge rate than NiMH batteries.
These are made in various sizes and capacities, from portable sealed to large fanned cells used for standby power and motor power. Smaller packs are used in portable devices, electronics, and toys, while larger packs are used in aircraft starting batteries and electric vehicles.
#3 Nickel-metal Hybrid Batteries (Ni-MH)

It is a rechargeable battery used in everyday electronic devices such as smartphones, laptop computers, and portable power tools. In this type, the chemical reaction at the positive electrode is similar to that of a nickel-cadmium cell, with both using nickel oxide hydroxide.
Nevertheless, the negative electrodes use a hydrogen-absorbing alloy instead of the cadmium that is used in NiCd batteries. This battery finds application in high-drain devices due to its high capacity and energy density. They are generally used as an alternative because they have a slightly lower but generally compatible cell voltage.
Read Also: Different Types Of CNC Machine [Complete Guide]
#4 Lithium-ion Batteries (Li-ion)

These types of batteries are composed of cells in which lithium ions move from the negative electrode through the electrolyte to the positive electrode during discharge and back when it’s charging. Lithium-ion batteries are used in heavy electrical current usage devices such as remote car fobs.
These are widely used batteries that are commonly found in laptops, mobile phones, cameras, etc. Lithium-ion batteries typically have a higher energy density, little or no memory effect, and lower self-discharge than other battery types. They have a longevity of 300 to 500 charge cycles or about two to three years.
#5 Alkaline Batteries

Alkaline batteries convert chemical energy into electrical energy by using manganese dioxide as the positive electrode and a zinc cylinder as the negative electrode to power an external circuit. The rechargeable alkaline battery is designed to be fully charged after repeated use.
It can be used for high- and low-drain devices but can wear out quickly in high-drain devices such as digital cameras. These batteries have a higher energy density and longer life, yet provide similar voltages as zinc-carbon batteries. It can be hazardous to recharge disposable alkaline batteries, so the user should look closely at its label.
#6 Zinc Carbon Batteries

A zinc-carbon battery provides a direct electric current from the electrochemical reaction between zinc and manganese dioxide in the presence of an electrolyte. These are found in appliances throughout the home, such as the remote control running the thermostat.
They typically offer higher capacity and lower internal resistance than alkaline batteries. In addition, they have improved low-temperature performance, improved leakage resistance, and low self-discharge. Zinc-carbon batteries have a short life cycle and are best suited for low-drain devices.
Read Also: What are the types of capacitors? Their Working & Uses
#7 Coin Cell Batteries

A coin cell battery is a small single-cell battery usually shaped as a squat cylindrical in diameter to resemble a button. These types of batteries have a separator that technicians contact an electrolyte between them, and control the flow of ions that create electricity.
They have a long service life and are found in small portable devices such as watches and pocket calculators. It is made of stainless steel that forms the cell’s lower body and positive terminal and a metallic top cap forms the negative terminal. Because they are so consistent and reliable, they are great for use in products that require long, continuous service.
#8 Zinc-air Batteries

Zinc-air batteries typically operate by oxidizing zinc with oxygen from the air. Since they are activated by air, they are ready for use when the oxygen interacts with the zinc in the battery. They have high energy density and are relatively inexpensive to produce.
They are available in a variety of sizes, from very small button cells for hearing aids to the large batteries used in film cameras. These types of batteries remain active until the power runs out, usually about three years. Benefits of this battery include flat discharge voltage, safety environmental benefits, and low cost.
#9 Sealed Lead-acid Batteries

It is a type of lead-acid battery in which the sulfuric acid electrolyte is condensed (thickened), so it cannot drain out. They are somewhat sealed but have vents if the gases are accidentally released by overcharging. This battery is designed to last up to 12 years.
It can be mounted in any position and does not require regular maintenance. It has a relief valve that is activated when the battery generates hydrogen gas. These types of batteries perform well at high load currents. They are found in motorcycles and all-terrain vehicles, wheelchairs, scooters, and boats.
Read Also: How does a DC motor works? [PDF]
How To Select A Battery?
There are only two features to consider when selecting a battery for your application which are performance and cost. But if we look a little deeper, there are a few more factors that go into choosing the right battery for your application.
Below are some factors to consider when selecting the right type of battery for your use:
#1 Energy Density
Energy density refers to the total amount of energy that can be stored per unit mass or volume. This determines how long your device remains on before it needs a recharge.
#2 Power Density
Power density refers to the maximum rate of energy discharge per unit mass or volume. For example, Low power is ideal for laptops, and i-pod. Whereas higher power is suitable for power tools.
#3 Safety
It is vital to ensure that the temperature at which you are making the device will work. In the case of high temperatures, some battery components will break down and may undergo exothermic reactions.
#4 Life Cycle Durability
The long battery life required for most applications needs the stability of the battery’s energy density and power density with frequent cycling (charging and discharging).
#5 Cost
It is important that the cost of your battery choice is proportional to its performance and does not abnormally increase the overall cost of the project.
#6 Charging Current
It is defined as the maximum current that can be applied to charge the battery. This is practically a maximum of 1A/2A that can be applied if a battery protection circuit is built-in but still 500 mA is the best range for a battery charge.
#7 Charging Voltage
Charging voltage refers to the maximum voltage that must be applied to the battery in order to charge the battery efficiently. Basically, 4.2 V considers the best charging voltage.
Wrapping It Up
As I already said, batteries are devices that accept, store, and release electricity on demand. There are many types of batteries available for consumer use, and each has different uses. It will continue to build the way we live as it plays a central role in enabling clean and renewable energy.
So for now, I hope that you have learned about the “Types of Transmission“. If you have any questions or doubts about this article, feel free to ask in the comments. If you got this article helpful, please share it with your friends.
Want free PDFs in your inbox? Then subscribe to our newsletter.
Download PDF of this article:
You might like to read more in our blog:
thank you very useful for my project
You’re welcome.
Hi,
I liked the types of batteries article, it was useful for me to know more about batteries, how to choose them and how to deal with them in the backup applications.
I’ll be glad to receive a pdf copy from this article.
Thank you in advance..
You’re most welcome. The PDF file has been sent to your inbox. Keep visiting 😉
I liked this article. Thanks
You’re welcome.
Sent me the download link please
The PDF file has been sent to your inbox.