In this article, you’ll learn what is thermodynamic cycles? how they work? and types of thermodynamic cycles and more.
Thermodynamic Cycles and Types
What is the thermodynamic cycle?
A thermodynamic cycle consists of a series of thermodynamic processes, which take place in a specific order, and the initial conditions are restored at the end of the processes. When the processes of cycles are outlined on the p-v diagram, they form a closed figure, each process described by its own curve.
Since the area under each curve is the work done to some scale. During each process, the work done during one cycle will be given by the area of the diagram as shown in the figure.
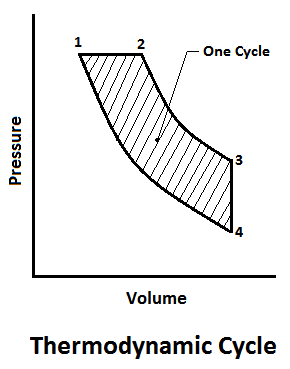
The thermodynamic cycles are very essential for the power developing systems (such as petrol engine, diesel engine, gas turbine etc.). These engines use a mixture of fuel and air for their operations. Since the mass of fuel used, as compared to the mass of air is very small, thus the mixture may be assumed to obey the properties of a perfect gas.
Read also: What is Thermodynamics and Laws Of Thermodynamics
A cycle, which requires four piston strokes and two complete revolutions of the crank is known as a four-stroke cycle. But a cycle, which requires only a two-piston stroke and one revolution of the crank, is known as a two-stroke cycle. When air is assumed to be the working substance inside the engine cylinder, the cycle is called an air cycle.
Let discuses next what are the types of thermodynamic cycles?
Classification of Thermodynamic Cycle
The thermodynamic cycles are classified into the following two types:
- Reversible cycle
- Irreversible cycle
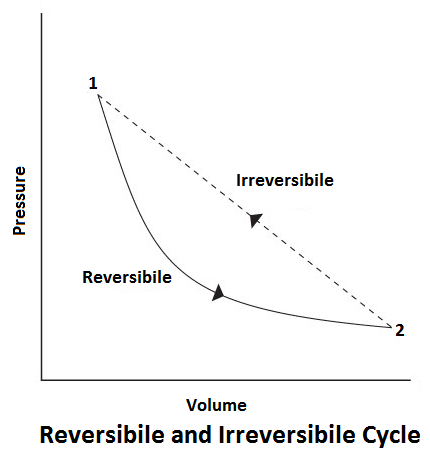
Reversible Cycle
In a reversible process, there should be no loss of heat due to friction, conduction or radiation, etc. The thermodynamically reversible cycle consists of reversible processes only. A reversible process is one which is performed at the end of the process, both the system and surrounding may be restored to their initial state.
For example, If during a thermodynamic process from state 1 to 2, the work done by the gas is W1-2, and heat absorbed is H1-2. Now, if by doing work W1-2, on the gas and extracting heat H1-2, we can bring the system back from state 2 to 1, the process is said to be reversible.
This process requires external power to start the mechanism according to the 2nd law of thermodynamics. A machine which operates on a reversed cycle is considered as a “heat pump”, for example, a refrigerator because it pumps heat from the cold body to the hot body.
Following are the conditions for reversibility of a cycle:
- The temperature and pressure of the working substance must not differ, from those of the surroundings at any stage in the process.
- All the processes, taking place in the cycle of operation, must be slow.
- The moving engine parts must be friction-free.
- There should be no loss of energy during the cycle of operation.
Note:
A reversible cycle should not be confused with a mechanically reversible engine. Steam engine cranks can be made to rotate in a reversed direction by mechanically changing the valve setting. But it does not reverse the cycle on which it works. A two-stroke petrol engine can be made to rotate in the reverse direction by altering ignition timing. But it also does not reverse the actual cycle.
Irreversible Cycle
As we have already discussed that whenever some change in the reverse direction reverses the process completely, it is known as a reversible process. But if the change does not reverse the process, it is called an irreversible process. An irreversible process causes heat loss due to friction, radiation or conduction.
In practice, most of the processes are irreversible to some degree. The main causes for the irreversibility are as follow,
- Mechanical and fluid friction,
- Unrestricted Expansion
- Heat transfer with a finite temperature difference.
Besides, the friction converts mechanical work into heat. This heat can’t supply back the same amount of mechanical work, which was consumed for its production.
Read also: Important Terms Used In Thermodynamics
Reversibility of Thermodynamic Processes
Now we shall discuss their conditions of reversibility.
1. Isothermal and Adiabatic
It may be noted that a complete process or cycle is only an ideal case. But in practice, complete isothermal and adiabatic processes are not achieved. However, they can be approximated. The simple reason for the same is that it is impossible to transfer heat at a constant temperature in case of an isothermal operation.
Moreover, it is also difficult to make an absolutely non-conducting cylinder in case of an adiabatic process. In actual practice, however, an isothermal operation may be approached if the process is so slow that the temperature remains, practically, constant.
Similarly, an adiabatic operation may be approached if the process takes place so quickly that no time is given to the heat to enter or leave the gas. In an isothermal and an adiabatic process as are taken as a reversible process.
2. Constant Volume, Constant Pressure and Constant pvn
We know that when the temperature of the hot body, supplying the heat, remains constant during the process, the temperature of the working substance will vary as the operation proceeds. In view of this, the above three operations are irreversible.
But, these can be made to approximate to irreversibility by manipulating the temperature of the hot body to vary so that at any stage the temperature of the working substance remains constant. In this way, the constant volume, constant pressure and constant pvn process are regarded as reversible processes.
3. Throttling
This process is irreversible, as there is always a loss of heat due to friction when the working substance passes through a narrow orifice.
The Difference Between Cycle and Engine
- In the theory of a heat engine, it is assumed that the working fluid is used again in the cylinder.
- We say that the fluid has undergone a cycle when it passes through different processes and returns back to its original state.
- In fact, the working fluid in an actual engine does not go through a full cycle and works on an open cycle.
- But for the purpose of simplicity in the analysis. We study the closed cycle (i.e. ideal cycle), which closely approximates the open cycle.
Working of An Ideal Engine
An ideal engine may be defined as a device, which develops work (i.e.power) continuously with the help of a working fluid, which undergoes some cyclic process. It is done with the help of a piston and cylinder as shown in Fig.
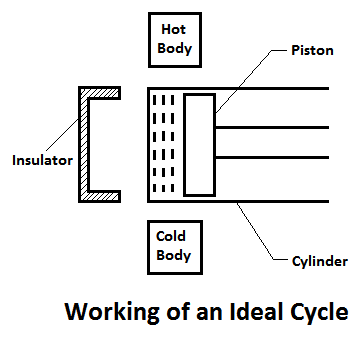
In general, the arrangement of piston and cylinder, of an ideal engine constitute the cycle by the following processes:
- The air in the cylinder is heated with the help of some external source. It processes temperature and pressure of the enclosed air.
- The air expands due to higher pressure to temperature. As a result of this, some work is done by the gas.
- The air rejects some heat to the external source. Then the air comes back to the original conditions.
- The air is compressed in the cylinder. As a result of this, some work is done on the air.
Note:
The scientists, working on the research and development of the engines, have focussed their attention mainly on the process of heating the enclosed air. Thus the various engines are classified according to the process of heat addition.
Types of Thermodynamic Cycles
Following are the types of thermodynamic cycles:
- Carnot cycle
- Stirling cycle
- Ericsson cycle
- Joules cycle
- Otto cycle
- Diesel cycle
- Dual combustion cycle
1# Carnot Cycle
In a Carnot cycle, the work material is subjected to cyclic operation. Carnot cycle consists of two isothermal and adiabatic processes. The working substance is air in a cylinder in which the piston moves. The engine must operate between two sources of infinite capacity. One at high temperature and the other at low temperature.
Read: Full notes on Carnot cycle with PV and TS diagram
#2 Stirling Cycle
Stirling cycle was produced by Robert Sterling which includes the original Stirling engine. Stirling cycle is a modified version of the Carnot cycle. It consists of two isothermal processes and constant volume processes. The last two processes are performed with the help of a refrigerator to make this cycle reversible.
Read: Full notes on Stirling cycle with PV and TS diagram
#3 Ericson Cycle:
The Ericson cycle is invented by John Ericson. This cycle is consists of two isothermal and constant pressure processes. It is thermodynamically reversible by the action of a regenerator. Ericson cycles are used in closed-cycle type gas turbines.
Read: Full notes on Ericson Cycle with PV and TS diagram
#4 Joules cycle
Joules Cycle is also known as the Brayton cycle. It is a thermodynamic cycle named after George Brayton. It describes the workings of a constant-pressure heat engine. Consists of two constant pressure and two adiabatic processes.
Read: Full notes on Joules cycle with PV and TS diagram
#5 Otto Cycle
An Otto cycle is a thermodynamic cycle that describes the working of a spark ignition piston engine. The modern petrol engines operate on Otto cycle and generally found in automobile engines.
Read Full: Wikipedia
#6 Diesel Cycle
The engine of heavy motor vehicles works mostly on the diesel cycle. Rudolf Diesel invented the diesel cycle in 1897. The diesel cycle differs from the Otto cycle in one case. This added constant pressure instead of constant volume. It comprises two adiabatic processes, one constant pressure heat addition process, and one constant volume heat rejection process.
Read: Full notes on Diesel Cycle
#7 Dual Combustion Cycle
The dual combustion cycle is the combustion of Otto and diesel cycles. It is also called the semi-diesel cycle because semi-diesel engines work on this cycle. In this cycle, heat is absorbed partially at constant pressure. It has two adiabatic, two constant volume, and constant pressure processes.
Important Terms for Thermodynamic Cycle
Though there are many terms used in a thermodynamic cycle, yet the following are important from the subject point of view;
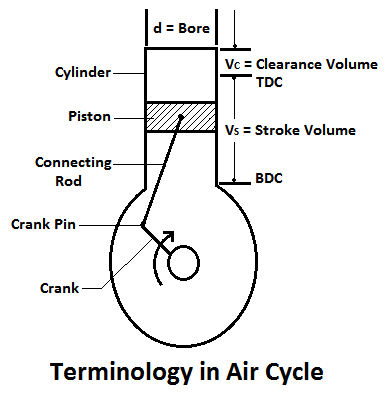
1. Cylinder Bore:
The diameter of the cylinder, in which the piston moves, is known as cylinder bore.
2. Stroke Length:
The piston moves in the cylinder due to rotation of the crank. Its extreme positions are known as a top dead centre (TDC) and bottom dead centre (BDC) respectively as shown in Fig. The distance between the two extreme positions is known as stroke length or stroke.
3. Clearance Volume:
The volume occupied by the working fluid, when the piston reaches the top dead centre, is known as clearance volume. It is generally denoted by (VC).
4. Stroke Volume:
The volume swept by the piston when it moves between the two extreme positions is known as swept volume, displacement volume, or stroke volume. Mathematically, swept volume,
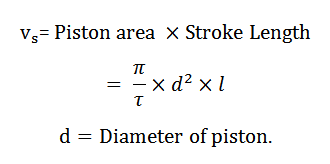
5. Full Cylinder Volume:
The volume occupied by the working fluid, when the piston is at the bottom dead center, is known as full cylinder volume. Mathematically, the full cylinder volume is equal to the sum of clearance volume and swept volume.
6. Compression Ratio:
The ratio of full cylinder volume to the clearance volume is known as the compression ratio. It is an important term is an engine. Mathematically, compression ratio,
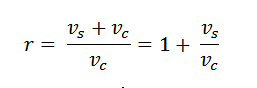
Note: This term is also called expansion ratio.
7. Mean Effective Pressure:
In fact, pressure in the cylinder keeps on varies with the position of the piston. For all sorts of calculations, we need the mean effective pressure, which may be defined as acting on the piston during the working stroke.
It will be able to do as much work as the actual varying pressures, generated during the cycle. It is the ratio of work done to the displacement volume.
Mathematically, mean effective pressure
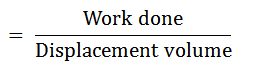
The Efficiency of a Cycle:
It is described as the ratio of work done to the heat supplied during a cycle. Mathematically, the efficiency of a cycle.
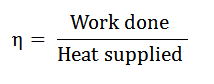
Since the work was done during a cycle is equal to heat supplied minus the heat rejected, the efficiency of a cycle, therefore, it is expressed as.
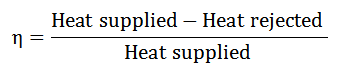
Note:
The above given efficiency is the theoretical efficiency of the cycle. Therefore it is known as theoretical thermal efficiency.
It does not take into account the practical damage done in the running of the engine.
To compare the efficiency of a thermodynamic cycle, the air is considered to be the working substance in the engine cylinder. Moreover, the air is allowed to act as a perfect gas. Thus, the efficiency obtained is known as air standard efficiency. It is also known as ideal efficiency.
That’s it. Thanks for reading, if you have a question about the “Types of thermodynamic cycles” ask in the comment section below. If you found this post helpful share it with your buddies.
Subscribe to our newsletter to get notification of new articles it’s free:
Download PDF of this article:
Read more on our blog:
What are some applications of the Stirling and Ericcson cycles?
Stirling and Ericsson cycles find applications in power generation, cryocooling, and heat pumps.
this is amazing explanation and need this PDF ,Full notes on Stirling cycle with PV and TS diagram ,Full notes on Ericson Cycle with PV and TS diagram and Otto cycle. i have exam next week. please !
The PDF file has been sent to your inbox. Please Checkout.
Hi,
could you send me the PDF of your different types of TC?
thanks in advance
You’re welcome. The PDF file has been sent to your inbox.
Which Types Of TDY cycle is used in IC engine
Otto cycle is the thermodynamic cycle which is most commonly found in automobile engines